Military Standard 105 (MIL-STD-105) is the grand daddy of sampling acceptance standards. The standard grew out of work done at Bell Labs in the 1930s and was first published during WWII. There were five updates to the standard, the last edition being MIL-STD-105E, published in 1989.
In 1995 the standard was officially cancelled when the military decided to go with civilian quality standards moving forward. Military Standard 105 lives on through its progeny, such as ANSI/ASQ Z1.4, ASTM E2234, and ISO 2859-1. There’s been an interesting interaction between civilian and military standards: Civilian organizations adopted military standards, then the military adopted civilian standards (which evolved from military standards).
From a statistical perspective, it seems a little odd that sampling procedures are given without as much context as an experiment designed from scratch might have. But obviously a large organization, certainly an army, must have standardized procedures. A procurement department cannot review thousands of boutique experiment designs the way a cancer center reviews sui generis clinical trial designs. They have to have objective procedures that do not require a statistician to evaluate.
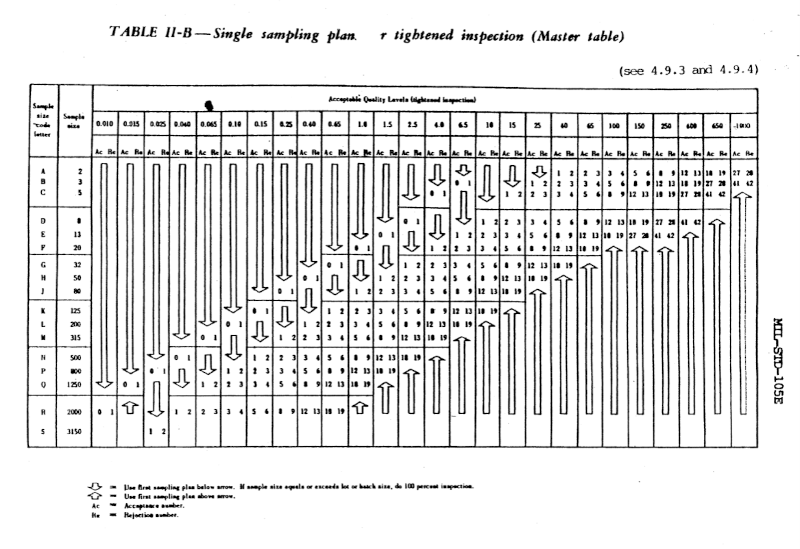
Of course manufacturers need objective standards too. The benefits of standardization outweigh the potential benefits of customization: economic efficiency trumps the increase in statistical efficiency that might be obtained from a custom sampling approach.
Although the guidelines in MIL-STD-105 are objective, they’re also flexible. For example, instead of dictating a single set of testing procedures, the standard gives normal, tightened, and reduced procedures. The level of testing can go up or down based on experience. During normal inspection, if two out of five consecutive lots have been rejected, then testing switches to tightened procedures. Then after five consecutive lots have been accepted, normal testing can resume. And if certain conditions are met under normal procedures, the manufacturer can relax to reduced procedures [1]. The procedures are adaptive, but there are explicit rules for doing the adapting.
This is very similar to my experience with adaptive clinical trial designs. Researchers often think that “adaptive” means flying by the seat of your pants, making subjective decisions as a trial progresses. But an adaptive statistical design is still a statistical design. The conduct of the experiment may change over time, but only according to objective statistical criteria set out in advance.
MIL-STD-105 grew out of the work of smart people, such as Harold Dodge at Bell Labs, thinking carefully about the consequences of the procedures. Although the procedures have all the statistical lingo stripped away—do this thing this many times, rather than looking at χ² values—statistical thought went into creating these procedures.
Related links
[1] This isn’t the most statistically powerful approach because it throws away information. It only considers whether batches met an acceptance standard; it doesn’t use the data on how many units passed or failed. The units in one batch are not interchangeable with units in another batch, but neither are they unrelated. A more sophisticated approach might use a hierarchical model that captured units within batches. But as stated earlier, you can’t have someone in procurement review hierarchical statistical analyses; you need simple rules.
